An Ex-citing future with hydrogen
Hydrogen: The secret elixir of life
DOI: 10.60048/exm20_08Outside expert circles, anyone claiming that hydrogen is the most important element for human life would probably be met with astonished faces. After all, hydrogen is almost entirely undetectable to our senses – most people have at least heard of this element in their chemistry lessons, but are only indirectly aware of its importance.
Aside from a few exceptions based on the effect of gravitation and radioactivity, hydrogen is the source of most primary energy that makes it possible for life as we know it to exist on our planet. The energy radiated by the sun, which controls the climate and nature on Earth, is generated by hydrogen-helium nuclear fusion taking place around 150 million kilometers away.
This energy powers plants' photosynthesis, providing us with food in the process. All raw materials that are considered fossil energy sources, i.e. coal, crude oil and natural gas, are remnants of photosynthetic processes that took place on our planet millions of years ago. Even "renewable" energy is the product of the sun's radiated energy and therefore, essentially, a product of the nuclear fusion of hydrogen molecules. If the sun's rays are used directly in solar thermal power plants in concentrated form to vaporise water and to drive steam turbines, the internal photoelectric effect is used in photovoltaic systems in order to generate voltages and therefore to produce electromotive force. However, the link between the fusion energy emitted by the sun and the use of kinetic energy by wind power plants is less obvious – but it is there. Wind is nothing more than airflow compensating for local pressure differentials, which in turn are caused by local differences in pressure. These are caused by the varying intensity of the sun's rays. When it comes to the use of biomass for energy, we once again return to the topic of photosynthesis.
However, hydrogen is the most important element in the development of humanity for more than just energy-related reasons. Think of the centuries-old human dream of flight, for instance – innumerable attempts at achieving this aim failed before French inventor Jaques Alexandre Charles succeeded in launching the world's first hydrogen-filled balloon in 1783. travelling 15 kilometres from the Champ de Mars in Paris to the village of Gonesse. Hydrogen has also enabled humanity to reach great heights, for instance by using hydrogen as rocket fuel to achieve our dream of space flight, and great depths, using hydrogen-powered fuel cells to explore the deep ocean.
Last, but certainly not least, hydrogen plays an important role as one of the two main elements in water – our true elixir of life.
We hope that the importance of hydrogen is now clear to all our readers. But why has this not taken root in our everyday conversations, assuming the same importance as other media such as air or water? The reason stems from the fact that hydrogen is not present as a standalone element in our living spaces – it is always incorporated into various chemical compounds. To release it as an element, a certain amount of energy must be used; if this energy is not present, the hydrogen quickly and spontaneously combines with other available elements such as oxygen. It is precisely because of this predictability that we are currently pinning our hopes for the energy revolution on hydrogen – but it also means that it must be classified as a dangerous substance.
Fossil raw materials – a blessing and a curse
The immense boom in western industrial countries, which began with the Industrial Revolution in the nineteenth century and brought prosperity to extensive areas of the world and Asia in particular, can – put simply – be traced back to the intensive use of fossil raw materials in combustion engines. This started with the combustion of coal to generate water steam, which powered steam engines, and led to combustion engines fired by diesel and petrol, and eventually to the extremely efficient gas turbines we use today.
Until then, humanity had to make do with the strength of its own muscles, aided by farm animals. Now, however, immense amounts of additional force are available for use to use. A simple calculation shows the scale of this increase. The energy content of a barrel of crude oil (159 litres), known as "barrel of oil equivalent" or "boe", amounts to 1628.2 kWh. A young, healthy person can generate around 100 kWh of power in one year (1). A single barrel of crude oil therefore contains the average annual working output of 16 people. Taking into account the fact that around 2.6 1012 barrels of crude oil have been extracted and used since the beginning of the Industrial Revolution, it is easy to estimate the enormous increase in usable energy in the form of fossil energy sources.
In recent years, these energy sources have fallen out of favour, primarily because of the generally accepted harmful effect that carbon dioxide, which is generated when combusting fossil raw materials, has on our climate. Additionally, it must be acknowledged that fossil raw materials will not last forever. For years, scientists have discussed when crude oil and natural gas will reach their peaks. Regardless of how realistic or accurate these estimates are, it is important that we are aware that extraction will become more and more difficult, since newly discovered deposits are always found in inhospitable regions such as deep in the ocean or the Arctic. Once we reach the point at which extracting the raw energy sources requires more energy to be used than the energy source contains, we will have reached a physical and economical limit that we cannot sustain for long.
It is therefore advisable for us to discover new sources of energy that will not only maintain the prosperity of the western world, but that also offer realistic development opportunities for less wealthy and developing regions, such as areas in Africa or South America.
Potential solutions are nuclear energy, which will not be discussed in further detail here, and renewable energy sources. Of these solutions, the possibilities offered by water power and biogenic raw materials have been largely exhausted.
In contrast, the use of photovoltaics, solar thermal energy and wind power offer significant opportunity for growth. Although these energy sources must be considered genuine alternative energy sources for the future, they also exhibit some serious disadvantages, in particular a low energy density and high volatility, i.e. periodic fluctuations in the supply. In order to be able to power a national economy such as Germany reliably and continuously using predominantly renewable energy, significant energy storage capacity and high energy density are required. Assuming a basic capacity of 70 GW that must be buffered over long periods of time, a storage capacity corresponding to a gigantic pumped-storage power plant with a volume equal to Lake Constance and a fall height of 800 m would be required (2). The properties of hydrogen mean that it is practically ideal as an energy source and storage medium; additionally, it can compensate for the two main disadvantages of volatile renewable energy sources, even if this comes at a cost.
The properties of hydrogen Ex-plained (3)
Under normal conditions, hydrogen is a colourless, odourless gas. Hydrogen is not toxic and causes no damage to the environment. Hydrogen is mostly present in nature as part of a compound; it is almost never found in pure form, for example as an unmixed gas. No other element has so many known compounds – the most frequent is water (3).
| Hydrogen (H2) | Methane (CH4) | |
|---|---|---|
| Density [kg/m3] | 0,08388 | 0,7175 |
| Molecule size [nm] | 0,276 / 0,106* | 0,324 |
| Ignition temperature in air [°C] | 585 | 540 |
| Max. flame speed [cm/s] | 346 | 43 |
| Ignition range in air [Vol.-%] | 4 - 73 | 5 - 14 |
| Heat conductivity [W/(m x K)] | 0,18339 | 0,0341 |
| Ignition energy [mJ] | 0,02 | 0,28 |
In a gaseous state and at a temperature of 0 °C, the density of hydrogen is 0.089 g/l. By comparison, the density of air is 1.29 g/l, 14 times heavier. This means that hydrogen has high buoyancy in the atmosphere, which can cause rapid evaporation in outdoor plants.
Due to the extremely small size of its molecules (see Table 1), hydrogen has high diffusivity and low viscosity. It can therefore diffuse into and penetrate other media very effectively. This poses particular challenges regarding the leak-tightness of hydrogen equipment, but these can be overcome through the use of suitable technology. We have a good understanding of the effect of hydrogen atoms creeping on metallic grain boundaries, which can lead to the metal becoming brittle as a result of subsequent formation of hydrogen molecules.
Many readers will remember from their chemistry lessons that hydrogen and oxygen can form an explosive mixture (oxyhydrogen). From a safety perspective, the extremely wide explosive range of this mixture, from 4 vol.% (lower explosion limit, LEL) to 77 vol.% (upper explosion limit, UEL), is of particular interest.
The minimum ignition energy of 0.02 mJ is among the lowest; there are only two other gases – acetylene and carbon disulphide – alongside hydrogen in the hazardous IIC ignition group. The relatively high minimum ignition temperature of 585 °C appears to be inconsistent with the low ignition energy. This discrepancy is due to the hydrogen's high conductivity. Since the net influx of heat is critical for an explosion to be triggered, only very hot surfaces are able to transmit enough heat into a hydrogen-air mixture to cause ignition.
Additionally, hydrogen's extremely high flame speed, which is eight times higher than that of a methane flame, also sets it apart from other explosive mixtures. This property can be used deliberately in rocket drives to generate an especially powerful impulse (mass x flame propagation speed). However, it can be incredibly destructive in the event of unwanted or uncontrolled hydrogen explosions. The following sections explain how such explosions can be effectively prevented and set out the connections between explosion protection methods and the use of hydrogen for civil applications.
Explosion protection for hydrogen applications
Overcoming the risks associated with hydrogen, particularly its extreme explosive tendencies in combination with a suitable means of oxidation, especially oxygen in the air, has been part of safety technology for decades. Large-scale technological use of hydrogen began with the development of the Haber-Bosch process for synthesising ammonia at the start of the 20th century. This application also demonstrates how incredibly beneficial this element is for humanity – synthetically produced ammonia enabled us to manufacture artificial fertilisers on a large scale for the first time, which significantly contributed to feeding the planet's continuously growing population. Currently, over 100 million tons of hydrogen are produced every year (5). As well as the production of ammonia, this vast quantity of hydrogen is also used primarily in refinery processes and for producing methanol.
Explosion protection has functioned reliably and safely in all these applications for many decades. Internationally, it is governed by the IEC 60079 and IEC 80079 standards series, for which the IEC's TC 31 Technical Committee is responsible. In most countries and regions, these standards have been converted into virtually identical regional and national standards and are observed in this form. Two very common types of protection, "flameproof enclosure" and "intrinsic safety", account for the aforementioned properties of hydrogen by categorising it as belonging to explosion group IIC. However, the planned significant expansion and consolidation of hydrogen use planned as part of the switch from fossil fuels to other primary energy sources bring new safety-related dimensions to consider. The German national hydrogen strategy therefore states (6): "In particular, there is a need for scientifically accepted measurement methods and assessment criteria that are enshrined in regulations, as well as internationally accepted technical standards and guidelines. What's more, a high level of safety must be guaranteed. Negative results and accidents can endanger the acceptance of hydrogen technology. It is important to build trust among users." The following sections explain the current situation using selected elements from the hydrogen chain.
Hydrogen production
Among the different methods for producing hydrogen, natural gas steam reformation is the most common, accounting for about 50% of production. Producing hydrogen from carbon is, as ever, very important. Since these conventional methods have no place in a future sustainable hydrogen cycle because of the carbon dioxide released as a result, it is enough to state here that tried-and-tested explosion protection concepts for large technological plants of this kind have existed for decades, based on the IEC 60079 standards series and primary explosion protection methods. Aside from this, the following document will focus on hydrogen production using electrolysis. This method is particularly suitable for the use of renewable energy sources, since, on one hand, it enables the aforementioned volatility of the energy supply to be buffered effectively and, on the other hand, it allows a medium with high energy density to be produced in the form of hydrogen. Currently, the proportion of hydrogen generated through electrolysis is fairly low, at around 5%, primarily due to the high production costs. The production of one kilogram of "green" hydrogen costs between three and five times as much as the same amount of hydrogen generated from natural gas steam reformation (3). The proportion of hydrogen generated through electrolysis using renewable energy in the future will depend predominantly on how energy prices develop, the efficiency of the electrolysis process and the development of natural gas prices. Global, mainly politically launched, schemes to develop hydrogen infrastructures, such as the "German hydrogen strategy" (6) or the "Roadmap to a US Hydrogen Economy" (7) will create suitable framework conditions for positive development.
At present, there are four basic electrolysis methods, which differ according to the process temperature and type of membranes used. The essential principle of electrolysis, regardless of the method, is that water can be split into its constituent parts – hydrogen and oxygen – when subjected to direct current. The membranes are essential. Firstly, they prevent the two gases from coming into direct contact and forming an explosive mixture. Secondly, they must be created so that ions can pass through them. Alkaline electrolysis is the most common and most technically sophisticated method. In this process, liquid mixed with sodium hydroxide is responsible for transporting the ions from the cathode to the anode. In both the PEM (Proton Exchange Membrane) and AEM (Anion Electrolysis Membrane) electrolysis methods, a solid is used as the membrane. All listed methods have process temperatures between 60 and 80 °C, meaning they are low-temperature electrolysis methods. The typical efficiency of these processes is between 65% and 82% (3). SOE (Solid Oxide Electrolysis) is the most important high-temperature electrolysis method. In this process, the membrane material is oxide ceramic. The process temperature is between 700 and 900 °C. Steam is split, rather than water in its liquid form. Although it takes more energy to reach the required process temperatures, the system achieves a high efficiency of 84% as it makes use of the waste heat (8).
There are a range of international standards that regulate the safety concerns relating to the most important elements of the hydrogen value chain, including electrolysis. ISO Technical Committee TC 197 initially published a Technical Report, ISO/TR 15916, (9); this report contains a comprehensive overview of the entire value added chain, including production, storage, transport and eventual use. It contains details about the relevant safety-critical properties of hydrogen and the specific associated hazards, in particular explosion hazards, and suggests measures to mitigate these risks. This non-binding Technical Report is a good introduction to this complex topic and provides readers with a deeper understanding of the specific implementation of the applied standards. Since 2019, international standard ISO 22734 "Hydrogen generators using water electrolysis – Industrial, commercial and residential applications" has applied to the electrolysis of water (10). It sets out detailed requirements for the design, construction, safety and operation of electrolysis plants. When it comes to safety, it must be emphasised that manufacturers of plants of this kind are required to perform a risk assessment in order to systematically record potential hazards, determine the probability of occurrence, and implement suitable countermeasures. For explosion protection, in particular, it is established below that zone classification using IEC 60079-10-1 (11) or appropriate national standards must be performed. This can be used as a basis for implementing suitable primary (preventing the generation of explosive atmospheres) and secondary (avoiding ignition sources in accordance with the IEC 60079 standards series) explosion protection measures. When doing so, it must be taken into account that the process may, under certain circumstances, cause oxygenation in the area around the plant.
In this situation, the primary explosion protection measures are intended to prevent the release of hydrogen by ensuring that the plant parts are sufficiently leak-tight. These measures are reinforced by monitoring the immediate surroundings using gas measurement equipment. Since leak-tightness is a critical aspect of most hydrogen plant safety concepts, it is of the author's opinion that it is absolutely necessary to include precise specifications for the specific implementation of leak-tight pipeline connections and equipment in international standardisation. This is particularly advisable in view of the specific properties of hydrogen molecules, which are described above. In Germany, the two categories "technically tight" and "permanently technically tight" have been successfully used for a number of years. The specific embodiments are defined in TRGS 722 "Preventing or restricting hazardous explosive atmospheres" (12). At a European level, this requirement has been taken into account in the latest version of EN 1127-1 "Explosive atmospheres – Explosion protection – Part 1: Basic concepts and methodology" in the form of Appendix B: Leak-tightness of devices (13). In any case, it must be established that there are significant differences between the German national specifications in (12) and European standard (13) with regards to the concepts and some details. At IEC or ISO standard level, there is currently no adequate specification at all.
Particular requirements exist for high-temperature electrolysis due to the process temperature (see above), which is far higher than the minimum ignition temperature of hydrogen.
At a national level, specifications for hydrogen plant explosion protection have existed for a long time. These include Section 1.2.7 "Plants for the production and use of hydrogen" within a collection of examples regarding explosion protection regulations (EX-RL) in DGUV Regulation 113-001 (14). Here, too, the leak-tightness of the plant, combined with suitable ventilation and gas monitoring measures and supplemented by organisational measures, is the central element of the safety concept. For electrolysis plants in enclosed rooms without supplementary technical and organisational measures, only the area under the ceiling is classified as Zone 2.
Hydrogen storage and transportation
Under atmospheric conditions, gaseous hydrogen has an extremely low energy density. In order to enable its use as an energy source under technically and economically acceptable conditions, it must therefore either be highly compressed (pressure between 700 and 900 bar) or liquefied (cryogenic storage below the boiling point of -253 °C). Combinations of both states, known as hybrid storage, are also common (3). In liquefied or highly compressed form, hydrogen has a very high energy density (energy content per unit of weight). Its calorific value is 33.33 kWh/kg – three times higher than that of crude oil, 11.6 kWh/kg. Even if we take into account that compressing hydrogen requires around 10 to 15% of the energy it contains, and liquefying hydrogen uses around 30% of this energy (3), a useable net energy density is obtained that is comparable to or, in the case of compression, higher than that of crude oil. At this point, it is worth once again remembering how incredibly important this energy-rich raw material has been to human progress throughout the past 170 years, as described at the beginning of this document. With the help of hydrogen, we can realistically expect this development to continue, even without the excessive and, eventually finite, exploitation of fossil reserves.
The compressor is an important piece of equipment for changing the state of hydrogen. A range of compressor types are in use, such as piston compressors, screw compressors and diaphragm compressors. Due to the safety and cleanliness requirements for hydrogen processing, these compressors must be extremely leak-tight and free from any sources of contamination (oil-free).
The hydrogen liquefaction process is performed step-by-step in a series of repeated compression, relief and heat exchange processes. In the first step, the gas is cooled to -40 °C (using ammonia as a coolant), before being cooled to -96 °C in the second step (using liquid nitrogen as a coolant). Lastly, in the third step, it is cooled to below -253 °C (using helium as a coolant) (15).
Currently, there is no internationally applicable standard that deals specifically with the compression or liquefaction of hydrogen. Technical Report ISO TR 15916 (9) deals with safety aspects relating to hydrogen liquefaction in a relatively general sense and contains a variety of related general safety requirements and design regulations, which must of course be observed for compressors and liquefaction plants as well. (10) sets out requirements for compressors for further processing of the hydrogen produced, in conjunction with electrolysers. Among other requirements, it stipulates that:
- They must be generally suitable for compression of gaseous hydrogen under the specified pressure and temperature conditions
- They must be equipped with suitable pressure relief systems
- An automatic emergency shut-off in the event of impermissible high pressures or temperatures, or insufficient suction pressures must be present
Information about the use of compressors for applications in hydrogen filling stations can be found in (16) and (17). The latter is described in more detail below.
Section 1.2.7.2 of the collection of examples from German explosion protection regulations (EX-RL) (14) contains specifications for indoor and outdoor compression of hydrogen. In enclosed spaces, technical ventilation combined with a gas warning system is stipulated as the first option. In this case, the room is classified as Zone 2 and only the area immediately around the drainage (condensate drain) is classified as Zone 1. In the event that the technical ventilation fails, the room in which the compressor is located must be classified as Zone 1 (15).
A range of suitable underground geological formations such as salt caverns, exploited oil and gas fields, or aquifers (former groundwater reservoirs) can be used for the large-scale, long-term storage of gaseous, compressed hydrogen. (3)
For short- or medium-term storage and transport, highly compressed, gaseous or cryogenic hydrogen – or sometimes solid hydrogen – can be used.
Other storage options, known as material storage, include storing hydrogen in solid bodies, liquids or on the surface of solid bodies. The most well-known of these is metal hydride storage. All material storage solutions are currently still in early development.
Special full-composite or steel-composite pressure tanks are used as high-pressure storage. For transport by truck, these containers are bundled on CGH2 (Compressed Gaseous H2) tube trailers and enclosed in a protective frame. Typical pressures in this case range between 200 and 250 bar (3). Larger transport volumes can be transported using Multiple Element Gas Containers (MEGCs), which use pressures of around 500 bar.
Liquid hydrogen is transported in tankers by road, sea or rail. The transported volumes are greater compared to gaseous hydrogen because the density of the liquid hydrogen is greater than that of hydrogen in compressed gaseous form. Nowadays, the technical solutions used for heat insulation and to cool the liquid hydrogen have been enhanced and developed to the point that large quantities of this liquid can be stored and transported for long periods of time without any significant evaporation losses. The transport containers and necessary accessories are subject to a comprehensive set of international standards set out in ISO TC 220 "Cryonic Vessels".
Pipelines represent an additional means of distributing large quantities of gaseous hydrogen. Transporting pure or high-percentage hydrogen requires specialised conduits, due to the unique properties of the gas. This is already possible in some countries; for instance, there are currently two pure hydrogen networks in Germany, which are operated by private companies and cover an area of several hundred kilometers(18). However, the cost of constructing a large-scale pipeline network to supply an entire nation do not seem justifiable in the near term. Mixing a tolerable quantity of hydrogen into existing natural gas networks seems to be a far more realistic approach. Analysis and initial practical results indicate that this is possible up to a hydrogen content of 10% without issues. Mixing hydrogen and methane reduces the carbon dioxide and nitrogen oxide emissions during combustion. However, the calorific value of the mixture, and therefore the energy efficiency of the system, decreases as well (19).
Handover to the end user – hydrogen filling stations
There are a variety of ways in which hydrogen, as a fuel, can be used to perform work. Its potential applications include heating buildings or powering ships, submarines, rail vehicles, trucks, cars and even aircraft. The provision of energy via uninterrupted power supplies and emergency generators is expected to become a significant field of use for hydrogen. The technology for these applications is currently at very different stages of development. While the concept of fuelling aircraft using hydrogen is still in its infancy, fuel-cell powered submarines and forklifts have become today's state-of-the-art technology. The use of hydrogen as a material in a range of industrial sectors is also developing at a considerable pace and is sure to grow further in the future. Alongside the aforementioned uses in the chemical and petrochemical sectors, new opportunities are opening up in other fields, such as replacing coal in steel production or cement production.
From an explosion protection perspective, at this point we must discuss hydrogen filling stations for fuelling road vehicles. Globally, the construction of wide-ranging networks to supply hydrogen-powered vehicles is underway. According to Wikipedia (accessed on 13th January 2021), in 46 of these networks were in operation in the USA and Canada at the start of 2021, while 110 such networks were in operation in Europe at the end of 2019. More progress has been made in Asia – in Japan alone, 160 hydrogen filling stations will be available by the time the Olympic Games are held in 2021.
The corresponding ISO 19880-1 international standard "Gaseous hydrogen – Fuelling stations – Part 1: General requirements" has been in place since 2020. This document, comprising more than 180 pages, sets out safety requirements for current public and private filling stations in precise detail.

Initially, the constructor/operator is required to perform a systematic risk assessment. The two comprehensive appendices, A and B, included at the end of this standard serve to aid the correct implementation of methods and content.
All potential sources of hazards must be taken into account in the risk assessment. The following plant parts, in particular, are defined as hazard sources from the start:
- Local hydrogen production units
- The entire external hydrogen supply system
- Compressors
- Tanks
- All non-welded pipeline connections
- The hydrogen distribution system to the vehicles
Hydrogen filling stations must be designed and constructed so that, in the event of intentional or unintentional release of explosive gases, under normal conditions the formation of explosive atmospheres is prevented, minimised, identified or controlled.
To prevent the release of impermissibly large quantities of combustible gases, automatic shut-off valves must be installed at suitable points within the plant.
The plant must be inspected in accordance with the IEC 60079-10-1 standard or sufficient national regulations and zone classification must be implemented on the basis of this inspection. As well as primary explosion protection measures such as guaranteeing leak-tightness and monitoring the atmospheres, measures to prevent potential sources of ignition must also be taken. This can be done using distances or limited access rights. Explicit reference is made to the ignition protection methods according to IEC 60079 ff. and IEC 80079 ff.
Constructive explosion protection is also provided. As a result, suitable relief devices are required in order to prevent hazards caused by overpressure.
The entire plant must be earthed and equipotentially bonded. Additional measures to prevent electrostatic charges must be taken.
Measures to minimise the harmful effects of events occurring near the filling station must also be implemented. This refers to fires in the surrounding environment, in particular.
Hydrogen compressors are also considered in detail. Alongside the aforementioned risk assessment, the following explicit requirements are set out for compressors:
- They may not contain any sources of ignition
- The vibration and movement of compressors must be compensated in order to prevent mechanical damage
- Temperature and pressure levels, as well as additional parameters that must be observed during liquefaction of hydrogen, must be suitably monitored
Extensive regulations have been set out regarding emergency shut down procedures.
Maintenance and inspection of the filling plant with regards to explosion protection must be performed in accordance with IEC 60079-17.
In Germany, there are currently no specific national regulations regarding explosion protection at hydrogen filling stations.
Reconversion
Electrical energy can be generated from hydrogen using fuel cells. Since this essentially involves reversing the process of electrolysis described above, it is sufficient here to establish that the working principles of this process can be categorised in the same way as electrolysis. For further details, see (3). Depending on the process, an efficiency between 30% and 70% can be achieved.
At an international level, explosion protection is regulated by IEC Technical Committee TC 105. At the start of 2021, eleven working groups were focusing on standardisation projects regarding a range of applications, from micro-fuel cells to drive units for drones. As a rule, these groups work on new standards, which are expected to be finalised in the next few years.
Further information can be found on the IEC website.
Already available standards include those relating to the safety of stationary fuel cell energy systems (20) and the safety of portable fuel cell energy systems (21).
Explosion protection is regulated in a similar way in both documents. The most important requirements in this context are:
- Hazards due to the accumulation of flammable atmospheres must be eliminated
- The limit for thinning for normal internal release is set at 25% of the lower explosion limit (LEL)
- All devices that are used within the thinning limit must correspond to zone classification in accordance with IEC 60079-10-1
- Surface temperatures must be limited to 80% of the spontaneous combustion temperature of the potential fuel-air mixture
- Suitable measures to protect against electrostatic charges must be implemented
- Safety devices that guarantee that the entire energy system exceeds the 25% thinning limit must be present.
Trace heating in accordance with IEC 60079-30-1 is specially required for stationary fuel cell systems. Protecting the entire hazardous area using a pressurised enclosure according to IEC 60079-2 is also an option.
As for all other elements of the hydrogen cycle, a comprehensive risk assessment must be performed and suitable measures must be implemented in order to reduce all potential hazards to an acceptable level of risk.
In Germany, there are currently no specific national regulations regarding explosion protection for fuel cell systems.
Prospects
Hydrogen is "in". More and more reports about planned or implemented plants for producing and distributing this gas are appearing everyday in the media. The stock prices of the most well-known hydrogen or hydrogen plant manufacturers have risen significantly since 2019. Unlike the events at the start of the millennium, this time it seems as though this hype is here to stay for much longer. Taking into account the sheer scale of the work required to comprehensively redesign global power supply and mobility infrastructures, this is no surprise. Little by little, the public is becoming aware that the construction and operation of a large-scale hydrogen infrastructure will not come for free. Green hydrogen produced using renewable energy sources is currently still far more expensive than "grey" hydrogen, i.e. hydrogen obtained using natural gas. The safety requirements are no more strict than those already in place for fossil fuels – but certainly no less stringent, at least when it comes to explosion hazards. From a safety technology perspective, its more convenient properties, such as its high volatility as a result of low density, are balanced out by less favourable properties such as its extremely low minimum ignition energy and high diffusion coefficient. The process industry has years of experience dealing with the explosion hazards involved in working with hydrogen; it is clear that many well-established regulations from the process industry will prove suitable for application in new hydrogen cycles and applications. However, there are also new circumstances to consider. In the future, hydrogen will not only be operated in effectively isolated plants operated by trained personnel – it will be decentralised and available to the public. Electrolysis plants will be built near wind farms. Extensive hydrogen supply and filling networks will be established. What's more, the plants will need to continually grow, in order to guarantee the availability of storage for volatile renewable energy sources discussed at the start of this document. In Appendix I of the 12th German federal immission protection regulation (incident ordinance), the following threshold quantities are set out:
- Operating ranges in accordance with Section 1 para. 1 sentence 1: 5,000 kg
- Operating ranges in accordance with Section 1 para. 1 sentence 2: 50,000 kg
As a result, hydrogen plants that exceed the specified threshold value fall within the scope of the incident ordinance and must meet its strict requirements regarding plant safety. The production capacity of large electrolysis plants with a power range of 100 MW must, at a minimum, exceed the lower limit value.
In light of these developments, and those expected in the near future, it is therefore encouraging to see that ISO and IEC are working on many important aspects of safety technology throughout the hydrogen cycle in international standards. As mentioned above, some of these standards have already been published, while others are set to be finalised soon. The next logical step should be to collaborate with the European standardisation organisations CEN and CENELEC to convert these standards to European standards and incorporate them into national standardisation, in the same way as in other industries. On a national level, they will then need to be harmonised with existing ordinances and regulations. Comparing the requirements in the new international standards with the corresponding statements in German Ex regulations (EX-RL) (14) makes it clear that significant work is still required in this respect.
References
1. Der Mensch in Watt. [Online] [Zitat vom: 29. 12 2020.] www.wienenergie.at.
2. Methanol als Energieträger der Zukunft. Dr. Plass, Ludolf. Frankfurt : DECHEMA Kolloquium: Methanol Chemie- Rohstoff und Energieträger der Zukunft, 2014.
3. Shell Wasserstoff-Studie: Energie der Zukunft. s.l. : Shell, 2017.
4. CHEMIE.DE. Chemielexikon: Wasserstoff.
[Online] [Zitat vom: 30. 12 2020.] www.chemie.de.
5. Hydrogen: A Renewable Energy Perspective. Abu Dhabi : IRENA International Renewable Energy Agency, 2019.
6. Die Nationale Wasserstoffstrategie. Berlin : Bundesministerium für Wirtschaft und Energie (BMWi), 2020.
7. Roadmap to a US Hydrogen Economy: Reducing emissions and driving growth across the nation. s.l. : ushydrogenstudy.org, 2020.
8. Hydrogen THE RENEWABLE FEEDSTOCK AND ENERGY CARRIER.
[Online] [Zitat vom: 01. 01 2021.] www.sunfire.de/en/green-hydrogen.
9. Technical report ISO/TR 15916: Basic considerations for the safety of hydrogen systems Second Edition. Geneva Switzerland : ISO, 2015 .
10. ISO 22734: Hydrogen generators using water electrolysis - Industrial, commercial, and residential applications: 1. Edition. Geneva : ISO, 2019.
11. DIN EN IEC 60079-10-1: Explosionsgefährdete Bereiche-Teil 10-1: Einteilung der Bereiche - Gasexplosionsgefährdete Bereiche. Berlin : Beuth, 2016.
12. TRGS 722: Vermeidung oder Einschränkung gefährlicher explosionsfähiger Atmosphäre. Dortmund : Bundesanstalt für Arbeitsschutz und Arbeitsmedizin BAuA, 2012.
13. DIN EN 1127-1: Explosionsfähige Atmosphären - Explosionsschutz - Teil 1: Grundlagen und Methodik:. Berlin : Beuth, 2019.
14. DGUV Regel 113-001 Explosionsschutz-Regeln (Ex-RL). Berlin : DGUV Deutsche Gesetzliche Unfallversicherung, 2020.
15. Olah, G.A., Goeppert, A. und Surya Prakash, G.K. Beyond Oil and Gas: The Methanol Economy. Weinheim : Wiley-VCH, 2018.
16. ISO 19880 - 3 Gaseous hydrogen-fueling stations - Part 3: Valves. Geneva : ISO, 2018.
17. ISO 19880 - 3 Gaseous hydrogen-fueling stations - Part 1: General Reqirements. Geneva : ISO, 2020.
18. Regulierung von Wasserstoffnetzen Bestandsaufnahme. Bonn : Bundesnetzagentur, 2020.
19. Dörr, H. u.a. Untersuchungen zur Einspeisung von Wasserstoff in ein Erdgasnetz. energie | waser-praxis. 2016, 11.
20. DIN EN IEC 62282-3-100: Brennstoffzellentechnologien - Teil 3-100: Stationäre Brennstoffzellen-Energiesysteme-Sicherheit. 1. Berlin : DIN, 2020.
21. DIN EN IEC 62282-5-100: Brennstoffzellentechnologien - Teil 5-100: Portable Brennstoffzellen-Energiesysteme - Sicherheit. Berlin : DIN, 2019.
More Article
Investigation of hydrogen generation in subsea umbilicals

In a growing number of cases, a build-up of hydrogen has been detected in the junction boxes located above the surface of the water, where…
Ex in sight
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/user_upload/magazin/artikel/20_27_Ex_im_Blick/1_Ex_im_Blick_Teaser.jpg)
Incorrect storage of hazardous chemical substances and inadequate surveillance of storage areas all too often result in catastrophic…
The Need for Integrated Project Management

Reducing project management and development time alone has the potential to deliver 15 to 30 percent in cost savings
Static and dynamic material stresses acting on Ex "d" enclosures

Flameproof enclosures must be subjected to certain testing, including of their ability to withstand pressure
PLP NZ celebrating 45 years with R. STAHL

45 years ago, three things came together, R. STAHL, PLP (Electropar Ltd), and the willingness to adopt innovative new hazardous area…
Emergency lighting

Central battery systems as emergency lighting systems offer secure protection in the event of a power supply failure
Sensing nonsense: When appearances are deceptive

Process engineering systems are generally controlled by measuring process variables such as temperature, pressure, quantity, fill level or…
Digital support for visual inspections using deep learning

The use of deep learning models offers huge potential for reducing the error rate in visual inspections. Smart object recognition enables…
Lightning and surge protection in intrinsically safe measuring…

According to Directive 1999/92/EC [1], the user or employer are obliged to assess the explosion hazard posed by their system and they must…
Ex assemblies, Part 1

The discussion about Ex assemblies is as old as the EU ATEX Directive, and now dates back almost 20 years
How R. STAHL TRANBERG is Meeting the Digitalization Demands of…

Digitalization and the integration of data and solutions is playing a pivotal role in the shipping and maritime industries today, having a…
The "PTB Ex proficiency testing scheme"

The "PTB Ex proficiency testing scheme" (PTB Ex PTS) is a project that involves developing interlaboratory comparison programmes to assess…
Non-electrical explosion protection

Manufacturers and users must acquire knowledge on the subject of non-electrical explosion protection, in order to assess the application,…
Certification in South Africa

Certification in South Africa has certain key differences from international certification, e.g. IECEx or ATEX
Global conformity assessment using the IECEx system
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/_processed_/c/e/csm_Einstiegsbild_IECEx_Paragraf_38414b50d0.jpg)
IEC Technical Committee (TC) 31, tasked with developing a global conformity assessment system for explosion-protected products
A mine of experience in industry
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/_processed_/f/f/csm_Einstiegsbild_e_tech_IEC_6270d7c66a.jpg)
He takes over from Thorsten Arnhold, who chaired the System for the past six years
Conformity assessment in the USA
In contrast to the international IEC/IECEx community and the European Union, the conformity assessment landscape in the USA is very…
25 Years of the Zone System in the USA

In the area of explosion protection, the publication of Article 505 in the 1996 National Electrical Code (NEC®) was seen as a giant step…
An Ex-citing future with hydrogen
![[Translate to Englisch:] [Translate to Englisch:]](/fileadmin/_processed_/0/1/csm_shutterstock_1644506059_7d60f29da8.jpg)
Aside from a few exceptions based on the effect of gravitation and radioactivity, hydrogen is the source of most primary energy that makes…
Certification of Ex products
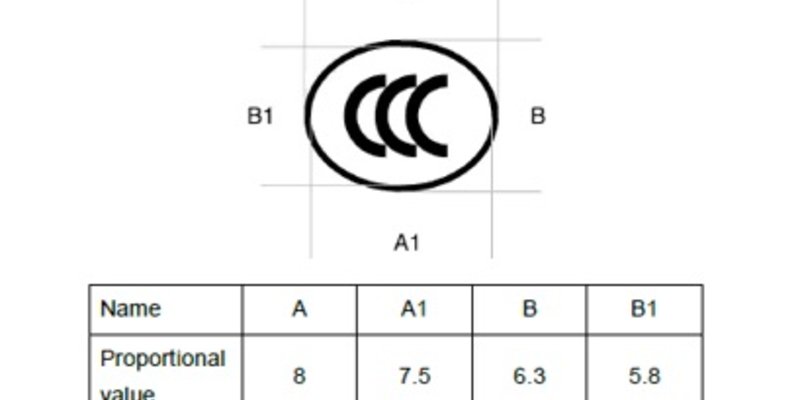
Since 1st October 2019, new CCC certification rules have been in place for Ex products sold in China


